
Abiotic stresses such as drought, salinity, and extreme temperatures threaten global crop yields. For optimum crop productivity, plants must efficiently combat these stresses. Rapid accumulation of proline under stress is a key feature and primary defense strategy in plants. Arabidopsis thaliana pyrroline-5-carboxylate synthase (AtP5CS), involved in proline metabolism, serves as the rate-limiting enzyme for de novo proline synthesis in plants. AtP5CS contains both glutamate kinase (GK) and glutamate-5-semialdehyde dehydrogenase (GPR) domains.
Recently, Professor Liu Ji-Long’s team at the School of Life Science and Technology (SLST), ShanghaiTech, unraveled the metabolic filament structures of the pivotal enzyme, AtP5CS, by utilizing cryo-electron microscopy and AlphaFold2, a protein structure prediction tool, representing a significant progress in plant stress biology. The study, titled “Dynamic Arabidopsis P5CS filament facilitates substrate channeling”, published in Nature Plants, demonstrates the unique mechanism for the efficient catalysis of AtP5CS, shedding light on the intricate mechanisms underlying plant proline metabolism and stress response.
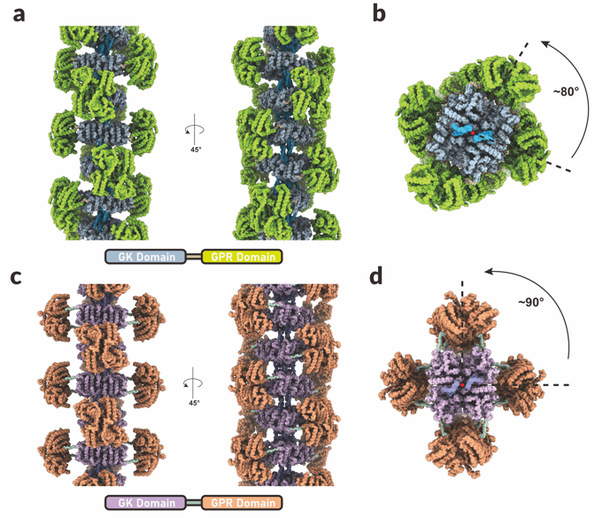

Figure 1: Metabolic filament structure models of AtP5CS1 and AtP5CS2
(a) Main and side views of the metabolic filament of AtP5CS1
(b) Top view of the metabolic filament of AtP5CS1
(c) Main and side views of the metabolic filament of AtP5CS2
(d) Top view of the metabolic filament of AtP5CS2
In this original study, researchers experimentally validated the importance of the metabolic filament structure of AtP5CS for its efficient catalytic activity. This unique filamentous structure is crucial for the transportation of the fragile intermediate product, L-Glutamic acid 5-phosphate (G5P), generated during the catalytic process of AtP5CS. By combining dynamic filament structure analysis with enzymatic biochemical experiments, the researchers proposed a novel model that reveals the intricate interplay between the catalytic process of AtP5CS and its filamentation.
On one hand, the catalytic reaction of AtP5CS promotes the formation of the filamentous structure. In the absence of substrate in the apo state, specific amino acid residues attract AtP5CS tetramers to aggregate, and upon substrate binding or catalysis, these tetramers begin to form stable filamentous structures. The formation and extension of this structure are achieved through the iterative interplay between tetramer attraction and catalytic processes.
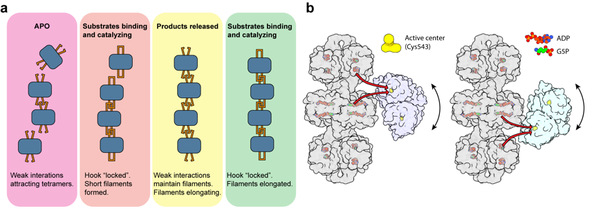
Figure 2: Dynamic AtP5CS metabolic filament promotes transport of fragile intermediate products
(a) Assembly model of the AtP5CS metabolic filament
(b) Mechanism of substrate channeling facilitated by the metabolic filament
On the other hand, filamentation of AtP5CS is crucial for enhancing the efficiency of transferring the fragile intermediate product G5P, which aids the entire catalytic process. Within the filamentous structure of AtP5CS, significant flexibility is observed between the two functional domains, GK and GPR, with the GPR dimer capable of rotating significantly around the GK tetramer. This structural feature allows for a significant reduction in the distance between the active centers of GK and GPR in certain instances, thereby facilitating the efficient transfer of intermediate products. Additionally, the distance between the active site of GPR and the adjacent helical unit of GK is nearly identical, providing another possible route for product transfer. The filamentous structure of AtP5CS serves as a unique platform for the rotation of GPR dimers and the transfer of intermediate products.
SLST second-year PhD student Guo Chenjun, and SLST first-year PhD student Zhang Tianyi, are the co-first authors of the paper. Professor Liu Ji-Long is the corresponding author.
*This article is provided by Prof. Liu Ji-Long