
Mitochondria and chloroplasts are organelles that include their own genomes, which encode key genes for ATP production and for carbon dioxide fixation, respectively (Fig. 1). Mutations in mitochondrial DNA (mtDNA) can cause diverse genetic disorders and are also linked to aging and age-related diseases, including cancer. Targeted editing of organellar DNA should be useful for studying organellar genes and for developing novel therapeutics, but it has been hindered by lack of efficient tools for study in living cells.
Fig. 1 The carbon cycle mediated by mitochondria and chloroplasts.
The CRISPR system, which is widely used in gene editing of nuclear DNA, relies on a complex of Cas effector proteins and guide RNAs, which, however, are difficult to deliver into mitochondria, and that has hindered the application of CRISPR systems to mtDNA. Cytosine and adenine deaminases used in nuclear genome base editors strongly favor single-stranded DNA, which can be formed in the R-loop region of the target site by the CRISPR-Cas ribonucleoprotein complex, but not by TALE arrays or ZFP. Therefore, a deaminase that catalyzes the deamination of bases in dsDNA is needed to efficiently edit organellar DNA. Recently, CRISPR-free, protein-only base editors, such as double-stranded DNA deaminase toxin A (DddA)-derived cytosine base editors and adenine base editors, have been developed and they can enable targeted organellar DNA editing in human cell lines, animals and plants.
Recently, Associate Professor Chen Jia in SLST and Professor Jin-Soo Kim at National University of Singapore published a review article entitled “Base editing of organellar DNA with programmable deaminases” in Nature Reviews Molecular Cell Biology. Prof. Chen Jia and Prof. Jin-Soo Kim are the corresponding authors. The article describes in detail the development of base editor for organellar DNA and its application to mitochondrial DNA in animals and plastid DNA in plants, and discusses the precision and efficiency limitations of these tools, and proposes improvements for therapeutic, agricultural and environmental applications.
The review article begins with a detailed description of the development of mitochondrial base editing tools, which are currently categorized into three main groups: DddA-derived cytosine base editors, DddA-dependent adenine base editors, and strand-selective base editing with DNA nicking.
DddA, a bacterial intertoxin identified in Burkholderia cenocepacia, is a double-stranded DNA cytidine deaminase. The DddAtox can be split into two non-toxic halves fused with mitochondrial targeting signal sequences (MTS), and TALE arrays and uracil DNA glycosidase inhibitor (UGI), respectively, so that it comprised the first application of DddA-derived cytosine base editors (DdCBEs) for base editing of mitochondrial DNA (Fig. 2).
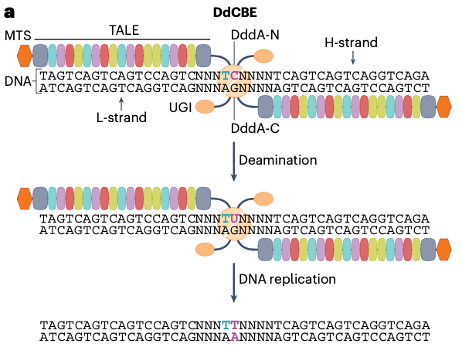
Fig. 2 The double-stranded DNA deaminase toxin A (DddA)-derived cytosine base editor (DdCBE).
The DddA-dependent adenine base editor is a fusion of TadA with DdCBE, which realizes the adenine deamination function of TadA with the help of the ability of DddA and constitutes the mitochondrial DNA base editors sTALED without UGI, dTALED, and mTALED, which are capable of A-to-G transition, and sTALED with UGI, which is capable of simultaneous C-to-T and A-to-G transition.
Strand-selective base editing with DNA nicking consists of the nickase (e.g., MutH) fused to a TALE array with a single-stranded deaminase (e.g., TadA8e-V106W) fused to a TALE array. Nickase exposes complementary single stranded DNA after forming a nick in a targeting site, allowing the single-stranded deaminase to function and thus mediate C-to-T or A-to-G editing effects.
The authors then described the applications and attempts to use mitochondrial base editors in animals, including mtDNA editing in mammalian embryos such as mice and rats, the use of AAV to mediate mtDNA editing in mice in vivo, and mtDNA editing in zebrafish. The animal models and editing tools used in each experiment, the targeting sites selected, and the final results such as efficiency and phenotype are also described and summarized in detail.
The authors further discussed the off-target mutations in mitochondrial gene editing and summarized the types and causes of off-target effects. In addition, the paper introduces the methods of detecting mitochondrial genome-wide off-target mutations and nuclear off-target mutations in mitochondrial gene editing in a comprehensive and detailed manner, and proposes the solutions to reduce off-target mutations.
Finally, the authors introduced the development and application of plant mitochondrial DNA and chloroplast DNA editing tools, and described their operation methods in detail, and discussed their applications in agriculture and environment.
**This news article is provided by Prof. Chen Jia